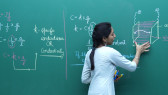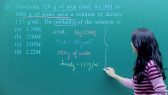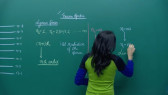Flat 50% off
BUY NOWEnds in
Published on 24, Jul 2019
We will discuss here Important theory and exam pattern question exercise about the topic Molar and Equivalent Conductance from chapter Electrochemistry for class 12 JEE main 2020 exam. This online video lecture prepared by best-experienced faculty of Misostudy in English (https://www.misostudy.com). Molar conductivity: is defined as the conducting power of all the ions produced by dissolving one mole of an electrolyte in solution. It is denoted by (lambda). ... But if solution contains 1 gm mole of the electrolyte therefore, the measured conductance will be the molar conductivity. Equivalent conductance: It is equal to the product of specific conductance (k) of the solution and the volume (V) of the solution that contains one gram equivalent of the electrolyte. Unit of spceific conductance k is Siemens / m. Now, required equivalent conductance unit is Siemens.m2/equivalent . Misostudy's courses will help students to boost for your JEE Main preparation to perform best in exams and achieve a good rank.
Related Videos